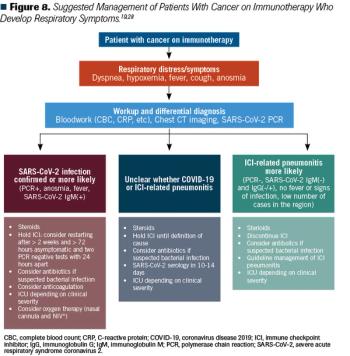
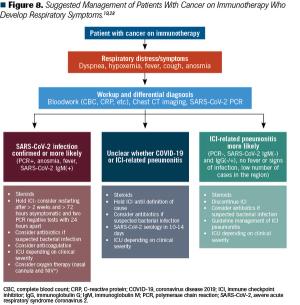
This article discusses the potential implications of using checkpoint inhibitors during the COVID-19 pandemic.

Your AI-Trained Oncology Knowledge Connection!


This article discusses the potential implications of using checkpoint inhibitors during the COVID-19 pandemic.
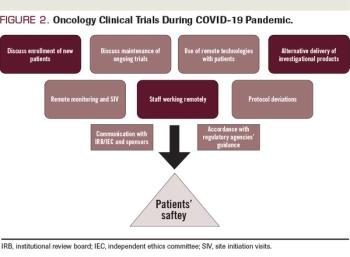
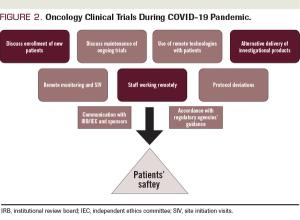
ABSTRACT The coronavirus disease 2019 (COVID-19) pandemic has rapidly spread all over the world in the past several months. No effective treatment for COVID-19 has been established. High transmissibility and considerable mortality rates have forced many national governments to implement quarantine measures. Many patients with cancer rely on clinical trials to receive their oncologic care, but the routine conduct of clinical trials has substantially changed because of the COVID-19 pandemic. The oncology research community should implement formal policies based on the guidance given from regulatory agencies, with the goal of minimizing the risks of COVID-19 infection while maintaining appropriate oncologic treatments for patients during this pandemic.
Published: September 22nd 2020 | Updated:
Published: July 15th 2020 | Updated: