Articles by George W. Sledge, Jr, MD
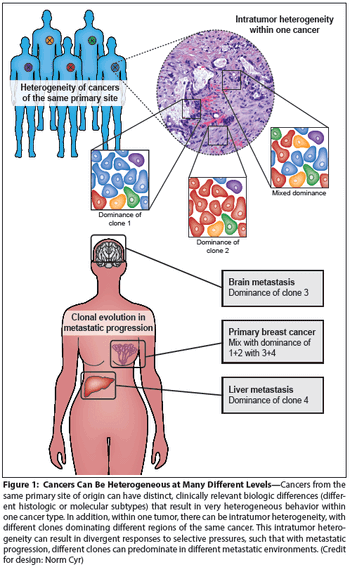
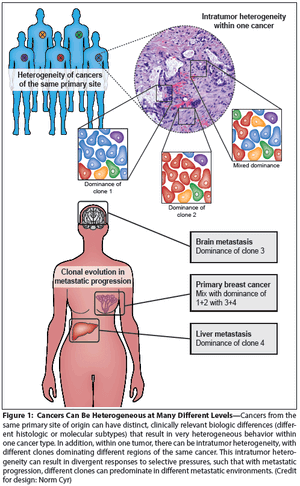
Cancer heterogeneity, long recognized as an important clinical determinant of patient outcomes, was poorly understood at a molecular level. Genomic studies have significantly improved our understanding of heterogeneity, and have pointed to ways in which heterogeneity might be understood and defeated for therapeutic effect.

In this video, Dr. Sledge discusses what the negative results of the ALTTO trial mean for women with HER2-positive breast cancer.

The strongest aspect of TCGA is that the data are publically available, fueling the input needed for unparalleled discovery. As the broader scientific community continues to analyze and integrate TCGA data with their own datasets, it is highly likely that breast cancer patients will benefit.

The treatment of microscopic metastatic breast cancer with adjuvant systemic therapy has undergone significant changes in recent years. At the same time, our understanding of the biology of breast cancer has also improved, predominantly as a consequence of data obtained from cDNA microarrays.

The past 2 decades of systemic therapy for breast cancer have beena period of monumental change, in terms of both theory and technology.Adjuvant therapy developed from two strands of research-one insystemic chemotherapy and one in hormonal therapy-both of whichwere aided by the application of higher statistical methodology to clinicaltrials. The agent with the single greatest public health impact inoncology has been tamoxifen, but problems with tamoxifen therapy ledto the development of the aromatase inhibitors, and further researchled to the use of hormonal therapy in a chemopreventive capacity. Theevolution of systemic chemotherapy for breast cancer has been an interplaybetween theory-driven approaches and new agents. By the late1980s, accumulating data revealed that overexpression of HER2 (erbB2)played an important role in a substantial portion of breast cancers,which prompted the development of trastuzumab (Herceptin), an agenttargeting HER2-positive disease. Determining HER2 status proved essentialto assessing patient eligibility for trastuzumab therapy. Decodingof the human genome and application of bioinformatics furtherrevolutionized the possibilities in breast cancer treatment.

Gemcitabine (Gemzar) and paclitaxel show good activity as singleagents and combined in metastatic breast cancer, and the combinationof paclitaxel/trastuzumab (Herceptin) has been shown to prolong timeto disease progression and survival significantly in this setting. Preclinicaldata indicate additive or synergistic effects of gemcitabine andtrastuzumab in HER2-positive human breast cancer cell lines. In aphase II trial, patients with HER2-overexpressing metastatic breastcancer who had received no prior chemotherapy for metastatic diseasereceived gemcitabine at 1,200 mg/m2 on days 1 and 8 and paclitaxel at175 mg/m2 on day 1 every 21 days for six cycles plus trastuzumab at aninitial loading dose of 4 mg/kg followed by 2 mg/kg weekly; patientswithout progressive disease after six cycles continued to receivetrastuzumab until disease progression. Overall, objective response wasobserved in 28 (67%) of 42 evaluable patients, including complete responsein 4 (10%) and partial response in 24 (57%); stable disease wasobserved in 7 (17%) and progressive disease was observed in 6 (14%).Median time to treatment failure was 9+ months. Median overall survivalhas not yet been reached, but is estimated at approximately 27months. Significant toxicities apart from neutropenia were uncommon.The triplet combination of gemcitabine, paclitaxel, and trastuzumab ishighly active and well tolerated in patients with HER2-overexpressingmetastatic breast cancer.

Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer
ByKathy D. Miller, MD,Judy Sisk, RN,Rafat Ansari, MD,Gary Gize, MD,Sreenivasa Nattam, MD,Kenneth Pennington, MD,George W. Sledge, Jr, MD A phase II trial evaluated the effectiveness and toxicity of combination paclitaxel (Taxol), gemcitabine (Gemzar), and trastuzumab (Herceptin) as first-line therapy for patients with newly diagnosed HER2-overexpressing