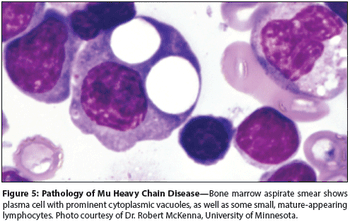
In 2004, multiple myeloma was diagnosed in more than 15,000 peoplein the United States and will account for approximately 20% of deathsdue to hematologic malignancies. Although traditional therapies suchas melphalan (Alkeran)/prednisone, combination chemotherapy withVAD (vincristine, doxorubicin [Adriamycin], and dexamethasone), andhigh-dose chemotherapy with stem cell transplantation have shownsome success, median survival remains between 3 to 5 years. Treatmentoptions for patients with multiple myeloma have increased in recentyears, with the promise of improvement in survival. New agents, suchas the proteasome inhibitor bortezomib (Velcade), the antiangiogenicand immunomodulator thalidomide (Thalomid) and its analogs, suchas lenalidomide (Revlimid), together with other small molecules, includingarsenic trioxide (Trisenox), and other targeted therapies, havebeen studied alone and in combination with other antineoplastic therapies,either as induction therapy prior to stem cell transplantation or inpatients with relapsed disease. Bortezomib recently was approved inthe United States for the treatment of multiple myeloma in patientswho have received at least one prior therapy. The use of bortezomibbasedregimens as front-line therapy as well as the use of other agentsin multiple myeloma remain under investigation, and approvals forboth thalidomide and lenalidomide are hoped for soon, with the overallprospect of patient outcome continuing to be increasingly positive.