Most adverse effects appear to be grade 1 or 2 among patients with hepatocellular carcinoma and other solid tumors associated with the MYC oncogene receiving OTX-2002 in the phase 1/2 MYCHELANGELO I trial.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

Most adverse effects appear to be grade 1 or 2 among patients with hepatocellular carcinoma and other solid tumors associated with the MYC oncogene receiving OTX-2002 in the phase 1/2 MYCHELANGELO I trial.

Neeraj Agarwal, MD, states that he is optimistic about the development of new treatment options including immunotherapies, CAR T-cell therapies, and antibody-drug conjugates for metastatic prostate cancer.
APOBEC mutational activity appears to be prognostic in patients with newly diagnosed multiple myeloma or precursor diseases who receive daratumumab quadruplets, according to Kylee Maclachlan, MBChB, PhD.

Clearance of an automated immunoassay allows practices to measure chromogranin A concentration in human serum, thereby tracking disease progression in those with gastroenteropancreatic neuroendocrine tumors.

The FDA has not requested any additional data or clinical trials supporting the supplemental biologics license application for an on-body injector presentation of pegfilgrastim-cbqv.
Pembrolizumab plus lenvatinib does not improve objective response rate compared with standard-of-care treatment among patients with metastatic non–small cell lung cancer in the phase 3 LEAP-006 and LEAP-008 trials.

Community health worker–led intervention may yield improvements in patient activation, hospice use, and total health care costs compared with usual care in those with newly diagnosed cancer.

Findings from the PRO-TECT trial suggest that patients and nurses are generally in favor of implementing financial toxicity screening into routine care for cancer.
Data from the NeoRes II trial caution against routinely delaying surgery for more than 6 weeks after patients with resectable locally advanced esophageal cancer receive neoadjuvant chemoradiotherapy.
Treatment with ibrutinib significantly reduces the risk of initiating next treatment in patients with chronic lymphocytic leukemia or small lymphocytic lymphoma compared with bendamustine/rituximab in a real-world setting.
A health-related quality of life analysis of the phase 3 NATALEE trial supports the risk/benefit profile of adding ribociclib to nonsteroidal aromatase inhibitors in patients with hormone receptor–positive, HER2-negative early breast cancer.

Findings from a cross-sectional study support monitoring puberal development to estimate probabilities of future fertility among male childhood cancer survivors.
Data from the phase 3 ASCERTAIN trial support the European Commission’s approval of oral decitabine and cedazuridine as a treatment for those with newly diagnosed acute myeloid leukemia.
Findings from a meta-analysis indicate that interventions for caregivers of patients with advanced cancer may improve self-efficacy and grief.

Combining regorafenib with nivolumab and chemotherapy appears to improve progression free survival at 6 months among those with advanced esophagogastric adenocarcinoma.
Findings from the phase 3 KEYNOTE-A18 trial support the supplemental biologics license application for a pembrolizumab-based regimen as a treatment for patients with newly diagnosed, high-risk, locally advanced cervical cancer.

Adding all-trans retinoic acid to non-intensive chemotherapy appears to produce higher toxicity in elderly unfit patients with acute myeloid leukemia harboring NPM1 mutations.

Data from the phase 3 LITESPARK-005 trial support the supplemental new drug application for belzutifan as a treatment for patients with advanced renal cell carcinoma.

KT-333 is under investigation as a treatment for adult patients with relapsed/refractory lymphomas, leukemias, and solid tumors in a phase 1 clinical trial.
Objective response rate appears to be numerically higher for patients receiving zanubrutinib for relapsed/refractory mantle cell lymphoma in the second-line setting than in later lines of treatment.

Findings from the phase 2 ALYCANTE trial support axicabtagene ciloleucel as a second-line treatment for patients with relapsed/refractory large B-cell lymphoma who are ineligible to undergo a transplant.

Data from a previous MPN LANDMARK survey highlight that many patients with polycythemia vera experience symptoms that lead to reduced quality of life.
Responses in patients with hormone receptor–positive, HER2-mutated breast cancer who crossed over to receive neratinib plus fulvestrant and trastuzumab support the necessity of neratinib in the triplet regimen.
Investigators report no differences in progression-free survival among patients with nonseminomatous testicular cancer who received surveillance or adjuvant chemotherapy in a cohort study.
Data from the phase 2 JACKPOT8 PARTB study support the new drug application for golidocitinib as a treatment for relapsed/refractory peripheral T-cell lymphoma in China.
Data from a phase 1/2 trial support the fast track designation for tulmimetostat as a treatment for patients with ARID1A-mutated advanced, recurrent or metastatic endometrial cancer.
Investigators report acceptable morbidity and health-related quality of life with neoadjuvant chemotherapy plus surgery and concomitant chemoradiotherapy in those with cervical cancer.
Allogeneic stem cell transplant should be strongly considered as a treatment for patients with mantle cell lymphoma who are adequately fit, according to Thomas E. Lew.
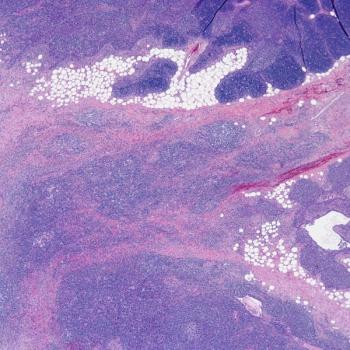
Investigators will assess AFM13 in combination with AB-101 as a treatment for those with relapsed or refractory Hodgkin lymphoma in the phase 2 LuminICE-203 trial.
Findings suggest the presence of different genetic pathways that may drive disparities in outcomes between Black and White patients with endometrioid endometrial cancer, according to Kristin Taylor, MD.