In a phase I study the targeted drug crizotinib, a small molecule inhibitor of anaplastic lymphoma kinase and met proto oncogene, delayed or eliminated signs of tumor growth in pediatric patients with aggressive cancers.

Your AI-Trained Oncology Knowledge Connection!


In a phase I study the targeted drug crizotinib, a small molecule inhibitor of anaplastic lymphoma kinase and met proto oncogene, delayed or eliminated signs of tumor growth in pediatric patients with aggressive cancers.
A new study has identified independent risk factors for the development of non-Hodgkin lymphoma (NHL) including high fetal growth, older age of the mother, low birth order, and male sex. A family history of NHL in either parent or sibling was found to be the strongest risk factor.
Researchers at the University of Pennsylvania have identified a protein that could be targeted to turn off B-cell lymphomas. The protein, CD19, was found to be a major regulator of B-cell neoplastic growth driven by the MYC oncogene.

Dr. Hoppe and colleagues present a strong case supporting the use of proton therapy (PT) for the treatment of patients with Hodgkin lymphoma (HL).

Proton radiotherapy is here to stay. Despite the high initial cost, the number of proton therapy machines in the United States and elsewhere is increasing rapidly.[1] The major questions now relate to defining and optimizing their appropriate use.

This review addresses the rationale and evidence for-and the challenges, cost implications, and future development of-proton therapy as an important part of the treatment strategy in Hodgkin lymphoma.

Leukemias and lymphomas are estimated to contribute up to 7% of all new malignant cases in the United States.[1]

The leukemias and lymphomas represent a group of heterogeneous myeloid or lymphoid clonal stem cell disorders with variable clinical presentation, pathological characteristics, prognosis and recommendations for treatment.[1]
The management of leukemias and lymphomas now includes the use of many targeted therapies. Nurses need to have an understanding of the targeted therapies and their side effects so they can appropriately manage the side effects that their patients with leukemias and lymphomas may experience.
Two researchers from the Mayo Clinic have published an editorial calling for new approaches in the treatment of AML, arguing that "using the same old drugs in different doses or different schedules is not going to cut it."

Cancer treatment is undergoing significant developments and entering the new golden era of genomics which has true potentials for the promise of personalized medicine. Large-scale sequencing is changing our understanding of malignant disorders particularly acute myeloid leukemia.
Inotuzumab ozogamicin has achieved a markedly long antitumor response in patients with refractory or relapsed indolent B-cell non-Hodgkin lymphoma (NHL) in an ongoing phase II study. Interim findings were reported by lead investigator Kenneth Luu, PhD, associate director of Pfizer global R&D.
A combination of TL32711, an investigational second mitochondrial-derived activator of caspases (Smac), and tumor necrosis factor-related apoptosis inducing ligand at low concentrations produced marked apoptosis in germinal center lines, with minimal to no effect for each agent alone.
Preliminary findings of a phase I/II randomized clinical trial indicate that SGI-110, a novel DNA methylation inhibitor, is safe, well tolerated and efficacious in patients with acute myelogenous leukemia.

Four publications on cancer treatment during pregnancy were published last week in the journal Lancet, serving as new treatment guidelines for chemotherapy and surgery in pregnant patients with solid tumors and hematologic malignancies.

Splenic lymphomas are a diverse group of lymphoid malignancies that have clinical behavior ranging from indolent to aggressive and that have both B-cell and T-cell histologies.

The results of the 2-year follow-up of the dasatinib DASISION phase III trial show the continued superiority of the drug compared to imatinib. The results provide further support for treatment of first-line chronic phase chronic myeloid leukemia patients that harbor the Philadelphia chromosome.

The phase III randomized RESORT (ECOG Protocol E4402) trial asked whether a maintenance schedule of rituximab every 3 months would lead to a superior disease control outcome compared to retreatment upon progression. The answer, presented this week at ASH, is no.
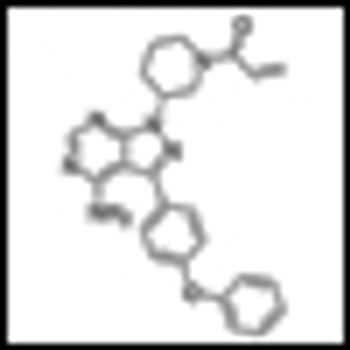
Updated findings from a multicenter phase Ib/II clinical trial suggest that the novel Bruton’s tyrosine kinase (BTK) inhibitor PCI-32765 may be an important new targeted treatment approach for patients with chronic lymphocytic leukemia.

The phase III trial comparing the use of gemtuzumab ozogamicin combined with chemotherapy to chemotherapy alone in newly diagnosed acute myeloid leukemia (AML) patients provides evidence that the combination treatment may be promising in this patient population.
Obinutuzumab (GA101) achieved higher response rates than rituximab in the first head-to-head trial of the two biologic agents in patients with relapsed non-Hodgkin lymphoma.

Andrew Evens, DO, MSc, deputy director for clinical and translational research and medical director of the Clinical Research Office at the UMass Memorial Health Care Cancer Center of Excellence, talks about his research on lymphoma during pregnancy.

CancerNetwork highlights four sessions--on anaplastic large cell lymphoma, lymphoma in pregnancy, follicular lymphoma, splenic marginal zone lymphoma--you won’t want to miss from this year’s ASH.

The US Food and Drug Administration has approved asparaginase Erwinia chrysanthemi for the treatment of patients with acute lymphoblastic leukemia, who have developed hypersensitivity to E. coli derived asparaginase and pegaspargase chemotherapy.
A survey of more than 500 long-term survivors of non-Hodgkin’s lymphoma (NHL) has revealed that more than one-third experience persistent or worsening symptoms of post-traumatic stress disorder (PTSD), with nearly 4 of 10 cancer survivors stating they still experience symptoms of PTSD more than a decade after their cancer diagnosis.