
Despite the widespread perception that herbal products are safe because they are “natural,” few of these products have been subjected to rigorous research. The potential risks associated with many herbal dietary supplements remain unknown.

Your AI-Trained Oncology Knowledge Connection!


Despite the widespread perception that herbal products are safe because they are “natural,” few of these products have been subjected to rigorous research. The potential risks associated with many herbal dietary supplements remain unknown.

According to published statistics, in 2008 approximately 66,120 new cases of non-Hodgkin lymphoma (NHL) were diagnosed and 19,160 lymphoma patients died from their disease despite currently available treatment.[1] Diffuse large B-cell lymphoma (DLBCL), the most common type of B-cell NHL, has an aggressive clinical course and, as demonstrated by gene-profiling studies, can be further divided into subgroups with distinct biologic characteristics and prognoses.[2]

In this issue of ONCOLOGY, Drs. Benjamin Besse and Thierry Le Chevalier have written a clear and concise review of the recent trials of adjuvant (postoperative) and neoadjuvant (preoperative) cisplatin-based chemotherapy for early-stage non–small-cell lung cancer (NSCLC)

Despite aggressive surgical management, 5-year survival rates of non–small-cell lung cancer (NSCLC) patients range from 73% for those with pathologic stage IA to 25% for those with stage IIIA.[1] Clinical or preoperative staging often underestimates the extent of the disease (particularly if positron-emission tomography and mediastinoscopy are not used), and the estimated survival rates for a given clinical stage are much lower than those for the corresponding surgical/pathologic stage.[1]

Besse and Le Chevalier provide us with an excellent and comprehensive review of available literature summarizing the current state of the art relating to both adjuvant and neoadjuvant (or induction) chemotherapy in resectable non–small-cell lung cancer (NSCLC).[1] They review the efficacy of adjuvant and neoadjuvant treatment in randomized clinical trials (RCTs) that employ cisplatin-based chemotherapy in stages I, II, and IIIA NSCLC, as well as meta-analyses of these RCTs.

The management of breast cancer in women under the age of 40 continues to challenge oncologists despite many recent therapeutic advances. The higher rates of breast cancer recurrence and death in this cohort strongly correlate with unfavorable clinicopathologic features

Breast cancer is the most common cancer in women, with over 180,000 new diagnoses of invasive disease annually in the United States, based on recent estimates.[1] Despite advances in therapy, over 40,000 women still die of breast cancer each year in the US.[1] While most women with breast cancer present with early-stage, potentially curable disease, young women face higher risks of recurrence and death compared to older women, which leads to challenges in selecting the optimal treatment strategy for these patients. The clinician is typically confronted with an otherwise healthy patient facing a life-threatening disease, and we are inclined to offer therapies with maximal benefit and minimal longterm toxicity, in the face of frequently inadequate or evolving data on how to achieve this.

Younger women with breast cancer present important management challenges due in part to differences in both tumor biology and individual patient factors. In his article, Peppercorn provides a comprehensive overview of these issues with a particular focus on questions surrounding systemic therapy options.

Breast cancer is the leading cause of cancer-related deaths in young women, and survival rates for young women with breast cancer are lower than for older women with breast cancer. This inferior survival is seen in spite of the fact that younger women often receive more aggressive therapy, as detailed in Dr. Peppercorn’s thoughtful review.[1]

The National Comprehensive Cancer Network (NCCN) has added everolimus (Afinitor) to the NCCN Guidelines for Kidney Cancer as a category 1 option for patients with metastatic renal cell carcinoma following failure of tyrosine kinase inhibitors such as sunitinib (Sutent) and sorafenib (Nexavar). This recommendation comes on the heels of the recent US Food and Drug Administration (FDA) approval of everolimus.

Over the next 20 years, the number of new cancer cases diagnosed annually in the United States will increase by 45%, from 1.6 million in 2010 to 2.3 million in 2030, with a dramatic spike in incidence predicted in the elderly (67% increase) and minority (100% increase) populations, according to research from The University of Texas M. D. Anderson Cancer Center.

A new federally funded University of Pennsylvania School of Medicine study aims to learn whether women at high risk of breast cancer can use exercise to meaningfully reduce their risk of getting the disease.

Reviewing treatment modalities for melanoma provides many sobering reminders that advances in our scientific understanding have not yet translated into meaningful clinical benefit. As clearly delineated by the authors, the “standard” treatment of dacarbazine chemotherapy has a poor response rate and lacks durability.

The article by Bhatia and colleagues focuses on the treatment of patients with metastatic melanoma using standard therapies, but it also includes a brief outline of recent treatment approaches using investigational agents. In addition, the authors describe prognostic factors for metastatic melanoma, highlighting the impact of the extent of tumor and the site of metastasis (eg, soft-tissue vs visceral metastases) on survival.
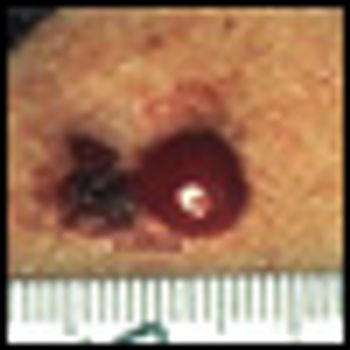
Metastatic melanoma continues to be a challenging disease to treat, with an estimated 8,420 related deaths in the United States in 2008.[1] The 10- year survival rate for patients with metastatic melanoma is less than 10%.[2] More than 3 decades after its initial approval by the US Food and Drug Administration (FDA) in 1975, dacarbazine continues to be the standard of care for most patients with this disease. High-dose interleukin-2 (HD IL-2 [Proleukin]), approved by the FDA in 1998 for metastatic melanoma, benefits a small subset of patients.