Oncology NEWS International
- Oncology NEWS International Vol 16 No 11
- Volume 16
- Issue 11
Dr. Norton hopes to weed 'molecular' tumor garden
Larry Norton, MD, is a gardener in the truest sense of the word. He is planting an idea (tumor self-metastasis) that could ultimately grow into a new way to understand and treat cancer. For decades, Dr. Norton, of Memorial Sloan-Kettering Cancer Center, accepted that cancer is a disease of cell division not unlike an acorn giving rise to an oak tree.
NEW YORKLarry Norton, MD, is a gardener in the truest sense of the word. He is planting an idea (tumor self-metastasis) that could ultimately grow into a new way to understand and treat cancer. For decades, Dr. Norton, of Memorial Sloan-Kettering Cancer Center, accepted that cancer is a disease of cell division not unlike an acorn giving rise to an oak tree. But these days, his garden is full of weeds, and that has sprouted the theory that each tumor may be a collection of individual seeds that grow wildly in a beda product of cell migration and not necessarily unstoppable division.
"Each weed bed is not particularly big or aggressive or fast-growing," Dr. Norton said, but together they can form a large aggressive tumor. "And each weed grows independently, throwing seeds to the wind that may, in turn, end up in the neighbor's garden."
If Dr. Norton is rightand scientists at Memorial and other cancer centers around the world are testing this ideait could explain why the current crop of medicines that target cell invasion are not curing cancer.
"I have spent most of my career developing therapies that target cell division," said Dr. Norton, who graduated medical school in 1972, a year after Nixon declared war on cancer. "But now I have come to believe that we may have been wrong in focusing our energies on cell division. It may not be a defining feature after all."
The Gompertzian curve
Dr. Norton is saying that biology and genetics may not be enough to explain the complex nature of tumorigenesis. In fact, he's using an old and powerful mathematical formulathe Gompertzian curvethat has been used to explain many events in nature.
He first used this model on the biological level to develop the idea of dose-dense therapy and now is using it on the molecular level to explain how tumors grow and metastasize.
In 1825, Benjamin Gompertz hypothesized that biological growth (such as population growth) follows a characteristic curve of rapid growth followed by slower growth, to an ultimate plateau. Applied to cancer, the Gompertzian curve suggests that malignant cells increase rapidly in number, then settle into slow growth up to a plateau. In this model, tumors can regress with treatment, but then, unless every single cancer cell is destroyed, the cancer cells naturally start increasing again in the rapid cycle mode.
Using this concept, Dr. Norton and his colleague Richard Simon, DSc, developed the Norton-Simon Hypothesis: Therapy results in a rate of regression in tumor volume that is proportional to the rate of regrowth that would be expected for an unperturbed tumor of that size.
A small tumor grows more rapidly than a larger one, and thus a single chemotherapy cycle can destroy more of these fast-growing tumor cells, but regrowth between cycles is also more rapid, resulting in a failure to completely eradicate the tumor.
Dr. Norton and his colleagues reasoned that dose density, focusing on scheduling and the amount of drug given, could help overcome the Gompertzian growth curve. By giving chemotherapy more often, they hoped to slow or prevent the rapid growth phase between chemotherapy cycles.
The first study of the concept (Bonadonna et al: JAMA 273:542-547, 1995) showed a lower risk of relapse in breast cancer patients receiving dose-dense sequential doxorubicin and CMF, compared with an alternating regimen.
Then, in a study by Dr. Norton and his colleagues (CALGB 9741), breast cancer patients with four or more positive axillary lymph nodes were randomized to receive chemotherapy at 2-week intervals or at conventional 3-week intervals. Use of the G-CSF filgrastim (Neupogen) allowed patients to receive the more "dense" regimen without developing dose-limiting neutropenia.
The results showed that those who had the drugs delivered in a more dense fashion had significantly better disease-free and overall survival, compared with those receiving conventional dosing regimens (Citron et al: J Clin Oncol 21:1431-1439, 2003), without increased toxicity.
"I think this has led to better chemotherapy, at least in breast cancer," Dr. Norton said. "The best dose level is not necessarily the maximum tolerated dose." In CALBG 9741, the better regimens were also the shorter ones, another patient benefit, he said.
Self-Metastasis
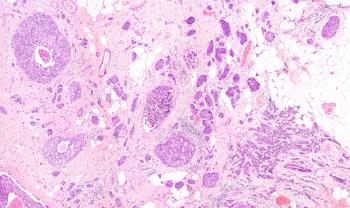
The success of dose-dense therapy led Dr. Norton to speculate about the molecular nature of tumors and whether the Gompertzian model applies. And here is where Dr. Norton throws in some gardening. He believes that a breast with cancer doesn't look like a complete and organized organ. It appears more like a bed of weeds rather than the oak tree, and each clump of weeds is a small and independent seed, or tumor.
This, he says, explains why the tissue laden with malignant tumor is much more dense than normal. It is anything but an organized mass, but rather a collection of small clumps of dense cell growth that together make up the tumor and account for its density. Further, he speculates, each tumor cell clump follows the initial rapid growth of the Gompertzian model, explaining the rapid growth of the tumor as a whole.
But how do these collections of small tumor clumps arise in the first place? In this model, cancer is a disease of self-seeding, and the tumor gives rise to its own weed clumps through self-metastases.
And just as a seed can travel on wind to a neighbor's yard, so can a cancer "seed" spread through the body and end up in a distant organ, or a completely new region of breast tissue, where the process of self-metastasis starts again.
In other words, large cancers don't metastasize because they are large, they are large because they are self-metastatic, Dr. Norton said.
Ultimately, designing drugs that target the seeding process may be the way to treat cancer, he said.
Math gives way to compassion
Science and mathematics aside, the lessons learned at the patient's bedside have far surpassed the lessons learned at the laboratory bench.
"We are human and vulnerable," Dr. Norton commented. "Doctors need the self-confidence and humility to deal with areas of ignorance as well as knowledge."
It is from this place of humility that Dr. Norton recognized that there are other ways to come at cancer, and it also led him to an important role in patient advocacy.
In addition to his daily duties as deputy physician-in-chief of breast cancer and director of medical breast oncology at the Evelyn H. Lauder Breast Center at Memorial Sloan-Kettering, Dr. Norton was president of the American Society of Clinical Oncology in 2001 and is scientific director of the board of the Breast Cancer Research Foundation.
"I am trying to cure cancer any way that I can," said Dr. Norton, who has used his voice for advocacy and to legislate for more research funding and access to care. He sees hope in the modern tools that are being used to understand how and why cancer cells behave the way they do.
But looking at the pieces of the puzzle won't tell the whole story. "What is the most important part of the airplane?" he asks. "It's the whole thing."
Articles in this issue
over 18 years ago
MT103, BiTE antibody, enters phase II testing for ALLover 18 years ago
Sides dig in as ESA policy debate heats upover 18 years ago
Ixempra gets ok for resistant breast cancerover 18 years ago
ASCO adds Oncotype DX to marker guidelineover 18 years ago
Accelerated approval for Tasignaover 18 years ago
Oxidative stress inducer ups survival in advanced melanomaover 18 years ago
FDA removes partial hold on Telcyta clinical developmentover 18 years ago
Should all HER2+ pts receive adjuvant trastuzumab?over 18 years ago
R-CHOP is standard of care for advanced DLBCL patientsover 18 years ago
FDA oks Velcade for patients with renal dysfunctionNewsletter
Stay up to date on recent advances in the multidisciplinary approach to cancer.