The use of statins, alone or in combination with metformin, was found to be associated with lower all-cause and prostate cancer mortality among high-risk patients, particularly in post-diagnostic settings.

Your AI-Trained Oncology Knowledge Connection!


The use of statins, alone or in combination with metformin, was found to be associated with lower all-cause and prostate cancer mortality among high-risk patients, particularly in post-diagnostic settings.
These findings highlight the need to raise public awareness of the importance of self-skin examination, and to encourage clinicians to have a lower threshold for undertaking skin examinations in bereaved individuals, according to the researchers.
In a paper published in The BMJ, the authors provide a summary for the diagnosis, treatment, and monitoring of thyroid nodules.
Phase II trial results found that phenelzine, a monoamine oxidase inhibitor typically used as an antidepressant, showed promise for patients with biochemical recurrent castrate-sensitive prostate cancer.

The new guidelines provide additional guidance for healthcare providers to better recognize and diagnose breast implant-associated anaplastic large cell lymphoma (BIA-ALCL).

The FDA approved isatuximab-irfc, in combination with pomalidomide and dexamethasone, for the treatment of adult patients with multiple myeloma who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor.
An interim analysis of the ongoing phase Ib study of resiniferatoxin, administered via epidural route in patients with intractable cancer pain, has produced positive data.

The FDA granted priority review to a biologics license application for tafasitamab in combination with lenalidomide for the treatment of patients with relapsed or refractory diffuse large B-cell lymphoma.

This study found racial and ethnic disparities in childhood and adolescent cancer survival for non-Hispanic black, non-Hispanic American Indian/Alaskan Native, non-Hispanic Asian or Pacific Islander, and Hispanic patients.
An artificial intelligence algorithm demonstrated better diagnostic performance in breast cancer detection compared to radiologists, who also saw improved performance when aided by the technology.
The key to predicting when liquid biopsy would produce clinically actionable information is the ability to image the blood brain barrier and macrophages, according to researchers.
Researchers suggested that patients with non-small cell lung cancer (NSCLC) who have higher measures of tumor mutations that appear in a blood test generally have a better clinical response to PD-1-based immunotherapy than those with a lower measure of mutations.

This study suggested that the National Comprehensive Cancer Network Distress Thermometer problem list does not easily identify concerns most associated with high distress and low quality of life in women with gynecologic cancers.
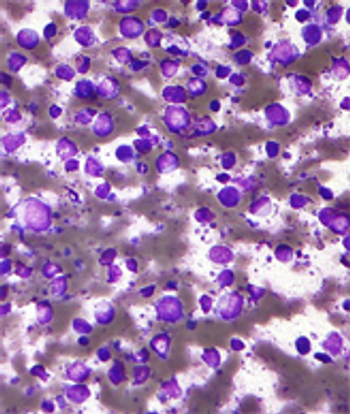
This recommendation was based on an analysis of patients with diffuse large B-cell lymphoma undergoing auto-HCT in which the addition of rituximab to the BEAM conditioning regimen had no impact on transplantation outcomes.
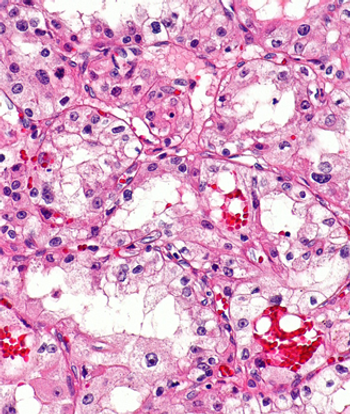
The findings reported in this study demonstrated a core dependency on HIF-2 in metastatic clear cell renal cell carcinoma and established PT2385 as a highly specific HIF-2 inhibitor.

The FDA Oncologic Drugs Advisory Committee voted 6 to 5 that ramucirumab plus erlotinib demonstrated a favorable benefit and risk profile for patients with untreated metastatic EGFR-positive non-small cell lung cancer.

In this study, researchers found that 59% of the participants reported at least 1 barrier to endocrine therapy adherence, though over half reported that taking endocrine therapy was a joint decision between themselves and their doctor.
This study provides the first evidence of the urinary TERT promoter mutations to be used as simple and cost-effective non-invasive biomarkers for the early detection of bladder cancer.

In this study, higher intakes of dairy milk were correlated with greater risk of breast cancer, when adjusted for soy intake.

The FDA approved a supplemental new drug application for neratinib in combination with capecitabine for the treatment of adult patients with advanced or metastatic HER2-positive breast cancer who have received at least 2 prior anti-HER2-based regimens in the metastatic setting.

A survey study documented slow progress in the advancement of palliative care programs and emphasized that access to timely palliative care continues to be a challenge for patients with cancer, especially those at non-National Cancer Institute cancer centers.
In this study, increased radiation dose was correlated with an increase in fatigue and dyspnea, as well as a decrease in physical activity, particularly in lung cancer or lymphoma.
Researchers found that poor infiltration of the tumor by immune cells, low activity from available T-cells, a lack of immune-stimulating neoantigens, and multiple immune-suppressing pathways all combine to dampen responses to immunotherapy in this disease landscape.

The FDA accepted a biologics license application for the fixed-dose combination of pertuzumab and trastuzumab with hyaluronidase, administered by subcutaneous injection in combination with IV chemotherapy, for the treatment of eligible patients with HER2-positive breast cancer.

The FDA accepted a supplemental new drug application for niraparib, treating women with advanced ovarian cancer who responded to platinum-based chemotherapy regardless of biomarker status, based on data from the PRIMA study.
These data, in combination with durable complete responses and overall survival data, suggested that tisagenlecleucel improved health-related quality of life in adult patients with relapsed or refractory diffuse large B-cell lymphoma who respond to treatment.
In this study, the skin cancer rates were higher among gay and bisexual men, compared with heterosexual men, but lower among bisexual women than heterosexual women.
In this study, researchers suggested that patients who play a more active role in making decisions about their prostate cancer surgery are less likely to experience “decision regret” about their choices.
A new tool, in combination with those already existing, could distinguish between patients with malignant and benign ovarian masses, as well as those with a benign ovarian mass and normal ovaries.

With February being National Cancer Prevention Month, here are the latest updates in cancer prevention.