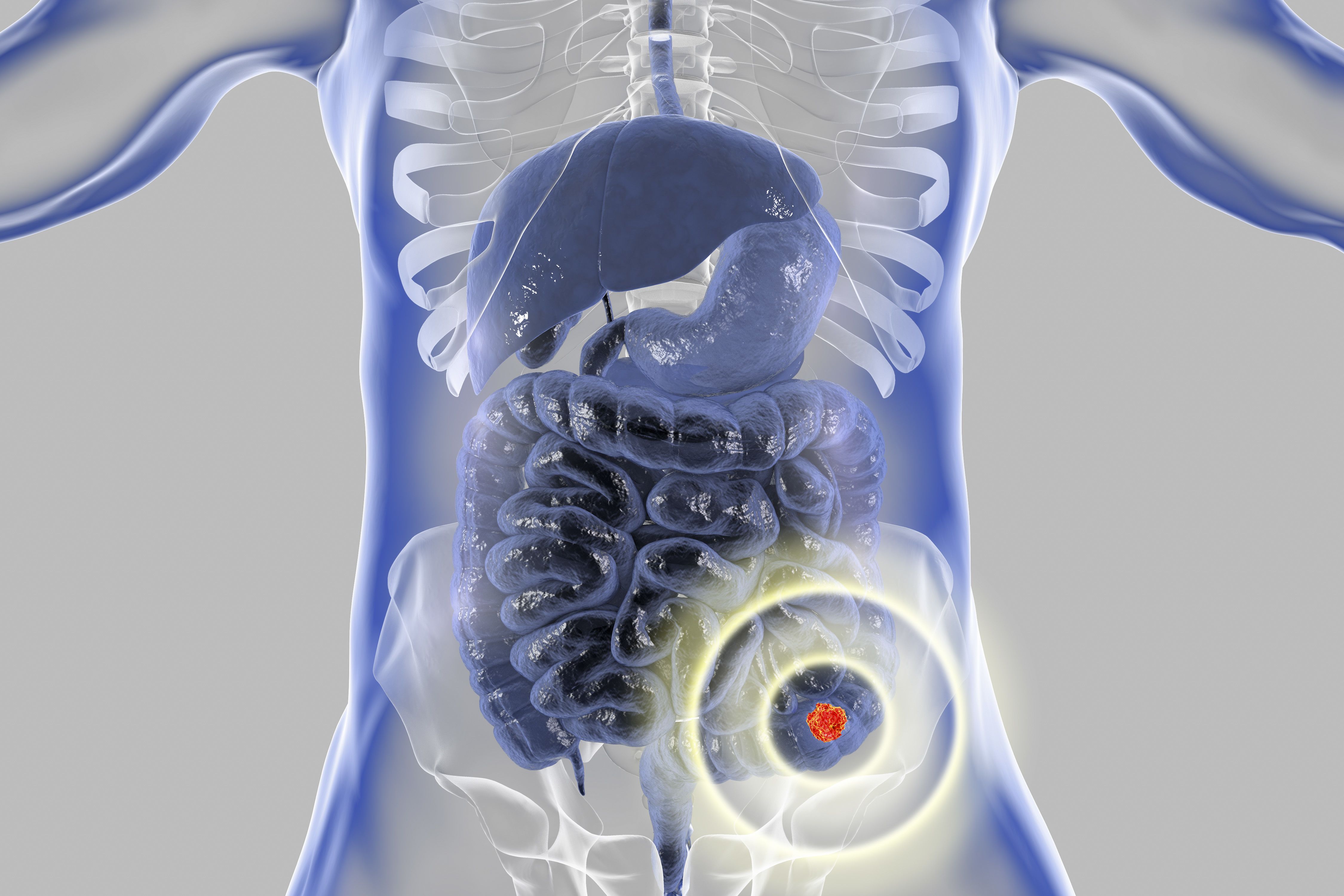
Colorectal Cancer
Latest News
Video Series

Latest Videos
Shorts
Podcasts
CME Content
More News

The Taiwan FDA has approved nivolumab/ipilimumab for patients with MSI-H/dMMR metastatic colorectal cancer based on data from CheckMate 8HW.

No serious adverse effects were observed with CBM588 in patients at risk of colorectal adenoma recurrence.

Larger-scale and longer-term studies could elucidate the mechanisms underlying quality of life benefits associated with resistance exercise in this group.

The addition of pelareorep to standard-of-care therapy in patients with KRAS-mutated microsatellite-stable CRC exhibited an ORR of 33%.
The anti–CTLA-4 antibody combination achieved an ORR of 34.8%, with 8 partial responses, in patients with pretreated microsatellite-stable mCRC.

With a median follow-up of 50.1 months, nivolumab plus ipilimumab achieved a median PFS of not reached compared with 60.8 months with nivolumab monotherapy in this CRC population.
Data from the STELLAR-303 trial support zanzalintinib plus atezolizumab as a potential chemotherapy-free option in previously treated metastatic CRC.

Findings from the DeFianCe trial support further development of sirexatamab in DKK1-high previously treated metastatic colorectal cancer.

Data from the INTERCEPT study support ctDNA clearance as a useful end point for potential benefit in studies assessing novel therapeutics.

Investigators are actively enrolling patients with locally advanced rectal cancer in the phase 1b FORTRESS trial evaluating NG-350A plus chemotherapy.
Neoadjuvant radiotherapy prior to surgery was associated with a 3-year OS rate of 88.5% in patients with locally advanced rectal cancer.


The test may help identify patients with microsatellite instability-high colorectal cancer who benefit from nivolumab therapy alone or with ipilimumab.

The performance of the latest Shield algorithm underwent validation in an expanded cohort of individuals enrolled on the ECLIPSE study.

Decreased MAPK signature and increased interferon gamma response signature were associated with sustained treatment benefit on serial evRNA profiling.

TAS-102 had a significant impact, achieving ctDNA clearance, in patients with colorectal cancer with ctDNA-defined MRD.

Developers launched a clinical laboratory-developed test version of Haystack MRD in late 2024 and are further expanding access for oncologists.

Significantly improved survival was observed with oxaliplatin among patients aged 60 to 70 years with stage III CRC but not among those older than 70 years.

Outcomes favoring robotic surgery for CRC may be influenced by patient selection factors, including clinical stability.
Data suggest that sotorasib plus panitumumab may represent a valuable new treatment option in this KRAS G12C–mutated colorectal cancer population.
An update of phase 1 data from the CTMX-2051-101 study is expected to be available by the first quarter of 2026.

Phase 2 CRDF-004 results revealed that adding onvansertib to chemotherapy/bevacizumab was well tolerated, with no unexpected toxicities observed.

Data from the phase 3 BEACON CRC trial support the approval of encorafenib plus cetuximab for this colorectal cancer population in China.

The FDA has waived the need for a botensilimab monotherapy arm in the phase 3 BATTMAN trial evaluating the combination in MSS metastatic colorectal cancer.
Subgroup data from BREAKWATER support cetuximab/encorafenib plus mFOLFOX6 as a new standard of care in BRAF V600E-mutated metastatic colorectal cancer.