Matthew T. Campbell, MD, MS, discusses CAR T-cell therapy and immunotherapy advances in renal cell carcinoma.

Your AI-Trained Oncology Knowledge Connection!


Matthew T. Campbell, MD, MS, discusses CAR T-cell therapy and immunotherapy advances in renal cell carcinoma.

Moshe Ornstein, MD, highlights recent treatment updates for renal cell carcinoma.


A reduced dosing duration of venetoclax plus hypomethylating agents may be appropriate for elderly patients with acute myeloid leukemia.

Data from the phase 1/2 ALKOVE-1 trial support the breakthrough therapy designation for NVL-655 in advanced ALK-positive non–small cell lung cancer.

Artificial intelligence use in prostate cancer encompasses 4 main areas including diagnostic imaging, prediction of outcomes, histopathology, and treatment planning.

The phase 2 DELLphi-301 trial showed durable response rates when tarlatamab was used to treat extensive-stage small cell lung cancer.

Safety data further support datopotamab deruxtecan as a new treatment option in metastatic hormone receptor–positive, HER2-negative breast cancer.

Results of the Sister Study cohort found an increased risk of ovarian cancer when enrolled patients used genital talcum powder throughout young adulthood.
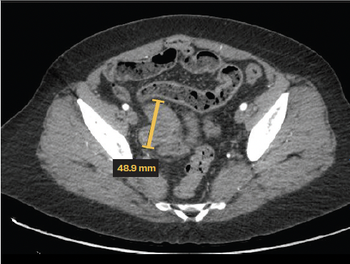
Learn more about a 56-year-old woman diagnosed with well-differentiated papillary mesothelioma, and how she was diagnosed and properly treated.


Data from the TRANSCEND-FL trial support the accelerated approval of lisocabtagene maraleucel in relapsed/refractory follicular lymphoma.

Alternative treatment strategies may be necessary for a high-risk population of patients with acute myeloid leukemia and residual FLT3-ITD.

Data from a phase 1/2 trial support the FDA breakthrough therapy designation for petosemtamab in recurrent head and neck squamous cell carcinoma.
Approval of the self-collection solution may reduce barriers to sample collection and increase access to cervical cancer screening.
![[SEBASTIAN KAULITZKI] - STOCK.ADOBE.COM](https://cdn.sanity.io/images/0vv8moc6/cancernetwork/3fbe7b21eef28f23798900792090beea0407b955-950x1030.png?w=350&fit=crop&auto=format)
Determining treatment options for patients with locally advanced rectal cancer after the PROSPECT trial data readout adds an important level to the decision-making process.
Patients with cancer who underwent surgery during the COVID-19 pandemic were also more likely to have respiratory complications.

The phase 3 KeyVibe-010 trial is unlikely to meet its primary end point of recurrence-free survival due to a high rate of treatment discontinuation.

While treatment centered around multiple drug options is common practice in oncology, the use of complementary and medicine alternative medicine is being introduced in the space.


The CheckMate 358 trial assessed various doses of nivolumab with or without ipilimumab for recurrent or metastatic cervical cancer.


The progression-free survival end point was not met in the CheckMate -73L trial assessing patients with unresectable stage III non–small cell lung cancer.

Gregory Charak, MD, discusses how modalities like laparoscopic surgery and neoadjuvant immunotherapy may benefit patients with colorectal cancer.

Phase 2 data support additional investigation of lifileucel as a treatment for patients with metastatic non–small cell lung cancer.


Although the sample size for this trial was small, data still highlight a potential benefit with nivolumab in dMMR uterine and ovarian cancers.