Prostate Cancer
Latest News
Latest Videos

CME Content
More News

Patients with BRCA-mutated metastatic castration-resistant prostate cancer can now receive treatment with olaparib plus abiraterone/prednisone or prednisolone following the combination’s approval by the FDA.

Fifteen-year follow-up data suggest the importance of considering the trade-offs between risks and benefits of active monitoring, prostatectomy, and radiotherapy for localized prostate cancer.

18F-rhPSMA-7.3 injection is now available to help identify PSMA-positive lesions during PET imaging for patients with metastatic or recurrent prostate cancer.

Synchronous disease appears to have a more hormone dependent transcriptional profile than metachronous disease, according to a retrospective review of patients with metastatic castration-sensitive prostate cancer.

Expert panelists consider the possibility of taking a break from treatment given a patient’s response to therapy along with other unique management strategies in the setting of metastatic hormone-sensitive prostate cancer.

In a third clinical scenario of metastatic hormone-sensitive prostate cancer, panelists discuss oligometastatic disease, radiation therapy, and the potential role for PSMA-PET scans.
Centering focus on a second clinical scenario of metastatic hormone-sensitive prostate cancer, key opinion leaders identify treatment options based on variations in presentation.
Shared insight on strategies to monitor patient response to therapy and select second-line treatment when hormone-sensitive prostate cancer progresses.

A post hoc analysis of the phase 3 TITAN trial highlights an association between PSA decline and survival among patients with metastatic castration-sensitive prostate cancer who were treated with apalutamide plus androgen deprivation therapy.

After nuancing quality of life data with chemotherapy in metastatic hormone-sensitive prostate cancer, panelists consider the value of multidisciplinary care.

Focused discussion on the respective roles of doublet and triplet regimens in patients newly diagnosed with metastatic hormone-sensitive prostate cancer.

Enzalutamide with or without leuprolide also reduces the risk of prostate-specific antigen progression in those with non-metastatic hormone-sensitive prostate cancer in the phase 3 EMBARK trial.
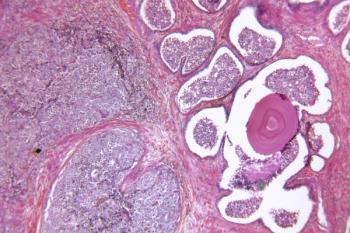
During the ODAC meeting, members voted to restrict the use of olaparib plus abiraterone and prednisone or prednisolone to patients with BRCA-mutated metastatic castration-resistant prostate cancer.

The addition of short-term androgen deprivation to dose-escalated radiation therapy did not yield a significant difference in quality-of-life outcomes vs radiotherapy alone for those with intermediate-risk prostate cancer, according to an expert from Henry Ford Health Cancer.

Panelists reflect on molecular markers and other clinical factors that aid in the selection of best therapy for a patient with metastatic hormone-sensitive prostate cancer.

Key opinion leaders in the field of prostate cancer management consider optimal molecular testing practices in a patient with metastatic hormone-sensitive disease.

The phase 3 SPLASH trial will assess the benefit of 177Lu-PNT2002 in patients with PSMA-expressing metastatic castration-resistant prostate cancer.
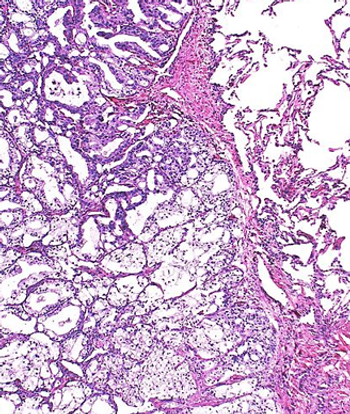
Data from the phase 3 MAGNITUDE study support the European Commission’s approval of niraparib plus abiraterone acetate dual action tablets in BRCA-mutated metastatic castration-resistant prostate cancer.

OTL78 appears to help in identifying prostate tumors, surgical margins, residual disease in the resection bed, and nodal metastases during PSMA-targeted fluorescence-guided surgery in those with PSMA-positive prostate cancer.
Dr Higano provides a timeline review of safety results for GnH agonists and GnRH antagonists including the recent prospective phase 3 PRONOUNCE study for prostate cancer patients with pre-existing CV disease, followed by closing thoughts on this video series.

Dr Higano explains how the use of ADT in patients with prostat ecancer may impact metabolic and cardiovascular (CV) risk factors, and shares best practices on optimizing risk assessment, mitigation, and monitoring for these factors.
A focused discussion on how ADT is used as the backbone of prostate cancer therapy across different prostate cancer risk groups and according to metastatic and castration sensitivity status.

Tia Higano, MD, FACP, provides a historical perspective on prostate cancer the use of androgen-deprivation therapy (ADT) and on prostate-specific antigen (PSA) in diagnosis and monitoring.

In addition to the benefit seen with hormone therapy plus metastasis-directed radiation in oligometastatic prostate cancer, use of intermittent hormone therapy may result in positive disease control and longer eugonadal testosterone intervals.

Factors including ARID1A mutations and tumor mutational burden appear to correlate with progression-free survival and overall survival following immunotherapy for advanced bladder cancer.