
Linda E. Carlson, PhD, RPsych, discusses the recent updates from SIO/ASCO to the anxiety and depression guidelines for patients with cancer.

Your AI-Trained Oncology Knowledge Connection!


Linda E. Carlson, PhD, RPsych, discusses the recent updates from SIO/ASCO to the anxiety and depression guidelines for patients with cancer.

Findings from an open-label trial support the FDA approval of inotuzumab ozogamicin as a treatment for pediatric patients with relapsed/refractory acute lymphoblastic leukemia.
“Ultimately, we’re going to be able to see more patients, offer more precise surgery more efficiently, and that means just better outcomes for our patients here in the Intermountain West,” said Hilary McCrary, MD, MPH
Investigators are evaluating IO-202 in patients with newly diagnosed chronic myelomonocytic leukemia as part of a phase 1 dose expansion trial.

Investigators are assessing avutometinib plus defactinib as a treatment for those with low-grade serous ovarian cancer as part of the phase 3 RAMP 301 trial.

A recent clinical quandary focused on the diagnosis and treatment of blastic plasmacytoid dendritic cell neoplasm in a resource-limited setting.

Investigators will assess A2B530 as a treatment for patients with germline heterozygous HLA-A*02–positive colorectal cancer expressing carcinoembryonic antigen in the phase 1/2 EVEREST-1 trial.
Findings suggest that harnessing the tumoral node microenvironment may improve the ability to predict extracapsular nodal extensions in patients with oropharyngeal carcinoma.

Denosumab biosimilars have been approved by the FDA as Wyost and Jubbonti across various reference indications.
Patients with factors such as lymphovascular space invasion or positive glossectomy specimen margins may be considered for adjuvant radiotherapy to optimize disease control of tongue squamous cell carcinoma.

Data from the phase 3 RUBY trial support the recommendation for dostarlimab plus chemotherapy for patients with advanced MSI-H/dMMR endometrial cancer.
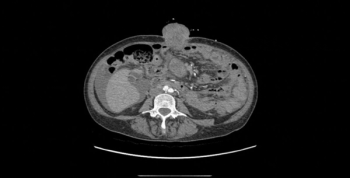
We present the case of a 51-year-old woman with metastatic International FIGO stage IIIC ovarian cancer who had delayed her therapy after initial laparoscopy due to COVID-19 infection and presented with an extreme case of surgical port metastasis.

Data from the TROPION-Lung01 and TROPION-Breast01 trials support the EU marketing authorization applications for datopotamab deruxtecan in non–small cell lung cancer and breast cancer, respectively.
A prospective phase 2 trial evaluated the use of de-escalation therapy for patients with p16-positive squamous cell carcinoma.
The role of multimodal approaches such as FDG-PET imaging may require further investigation in patients with human papillomavirus–positive oropharyngeal cancer, according to Samuel Regan, MD.

Julie M. Vose, MD, MBA, discusses the pros and cons of patients having early access to electronic medical records.
The use of postoperative adjuvant radiation therapy led to less weight loss in patients with HPV-positive oropharynx cancer, according to Wade L. Thorstad, MD.

The regulatory agency has cleared developers to continue patient enrollment in the phase 2 IOV-LUN-202 trial evaluating LN-145 in non–small cell lung cancer.

Amar H. Kelkar, MD, presented findings on the cost-effectiveness of transplantation therapy during the 2024 Tandem Meeting.

Rahul Banerjee, MD, FACP, highlights recent presented from the 2024 Tandem Meeting.

Noa Biran, MD, speaks about recently approved bispecific therapies and current trials that may impact the standard of care in relapsed/refractory multiple myeloma.

Robert A. Winn, MD, discusses financial initiatives for expanding access to anti-cancer treatment and how collaborations with professional organizations may spread awareness of cancer disparities.

Panitumumab plus modified FOLFOX6 appears to increase survival among patients with metastatic colorectal cancer and circulating tumor DNA that has no gene alterations in the PARADIGM trial.

Prophylactic cranial irradiation may not be worthwhile for treating patients with extensive-stage small cell lung cancer based on conflicting data, according to Gregory Peter Kalemkerian, MD.

FDA-approved immunotherapy options such as atezolizumab and durvalumab have produced substantial benefits in certain groups of patients with extensive-stage small cell lung cancer, says Gregory Peter Kalemkerian, MD.
Higher CA19-9 levels appear to correlate with increased recurrence and mortality for specific patients with pancreatic cancer who undergo surgical resection.

Frontline pembrolizumab with or without chemotherapy appears to remain a standard of care for patients with recurrent or metastatic head and neck squamous cell carcinoma based on data from the LEAP-010 study.

Data from the phase 3 PAPILLON trial support the FDA approval of amivantamab plus chemotherapy for patients with metastatic non–small cell lung cancer harboring EGFR exon 20 insertion mutations.

Results from a phase 1/2 trial show clinical activity of APG-115 in patients with p53 wild-type salivary gland cancer.

Results from cohort A of a pilot study of patients with HPV–associated oropharyngeal carcinoma did not meet its primary end point.