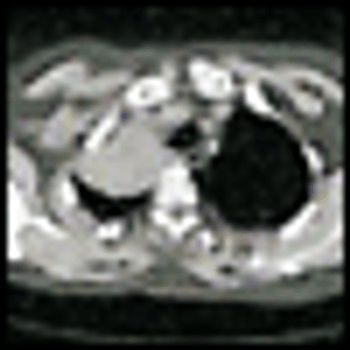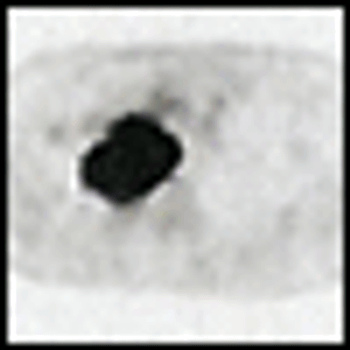
Because of the high rate of distant disease recurrence, the 5-yearsurvival of patients who have undergone complete surgical resectionof localized non–small-cell lung cancer (NSCLC) is approximately 50%.Initial results from early studies of adjuvant postoperative chemotherapyreported an adverse effect of alkylating agent and older chemotherapyregimens on survival. Cisplatin-based combinations were the first toshow a survival advantage. A 1995 meta-analysis of these studies suggesteda 13% reduction in the hazard ratio for death (HR = 0.87), leadingto a 5% survival benefit at 5 years. Still, these trials involved limitednumbers of patients (N = 1,394), and the results failed to reach statisticalsignificance (P = .08). Of the five largest subsequent randomizedtrials of platinum-based adjuvant therapy, three showed a significantsurvival advantage. Although it is impossible to determine the reasonsfor the differing outcomes of these studies, several key features distinguishthem, and the data suggest that medically fit patients with resectedstage IB or II NSCLC should be offered chemotherapy with a platinum/new drug combination.