
Unfortunately, although agents in the pipeline each extend life, none are curative. Therefore, physicians who investigate and treat mCRPC have two paths they can follow to further improve outcomes.

Your AI-Trained Oncology Knowledge Connection!


Unfortunately, although agents in the pipeline each extend life, none are curative. Therefore, physicians who investigate and treat mCRPC have two paths they can follow to further improve outcomes.

Node-positive prostate cancer without distant metastases (T any, N+, M0) currently is encountered rarely, primarily because of the shift to diagnosis at earlier stages, a result of widespread PSA testing.

In this review, we focus on the testosterone/androgen receptor pathway that is being targeted with potent new agents; we also discuss other important alternative biologic pathways that have given rise to new therapeutics that may attenuate prostate cancer growth, survival, and propagation.

The “experts” should maintain a stringent standard regarding what merits further development and reconsider carefully and critically the available data before committing to PARP inhibition, attacks on the PI3K pathway, and vasoactive agents in prostate cancer.

Current evidence for the management of lymph node–positive prostate cancer suggests both a disease-control and survival benefit to systemic ADT plus surgery and radiation.

From 2004 to 2009, use of advanced treatment technologies increased among prostate cancer patients with low-risk disease and high risk of noncancer mortality.
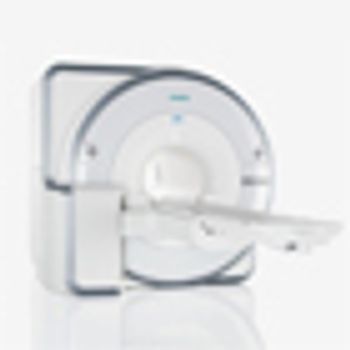
A new imaging approach, combining PET with magnetic resonance (MR), was shown to be more sensitive in detecting recurrent prostate cancer compared with PET/CT.

In a new study presented at the Endocrine Society’s annual meeting, researchers using animal models show that early BPA exposure increases prostate cancer risk.

A new study shows that men diagnosed with prostate cancer may do better by substituting carbohydrates and saturated fats with plant-based fats such as those found in nuts and olive oil.

In my experience, being treated for low-volume Gleason 6 tumors is the norm, not the exception, for men in the United States. Surveillance may be discussed as an option, but it is not taken seriously.

The concept of active surveillance is based on the observation that Gleason 6 (pattern 3) prostate cancer is an indolent condition that poses little or no threat to the patient’s life. Conservative management is thus appropriate for these patients.

Further analyses of data subsets from the ALSYMPCA study of the alpha particle-emitting isotope Ra-223 (Xofigo) were presented at ASCO, providing additional evidence of efficacy and safety of the recently FDA approved therapeutic agent.
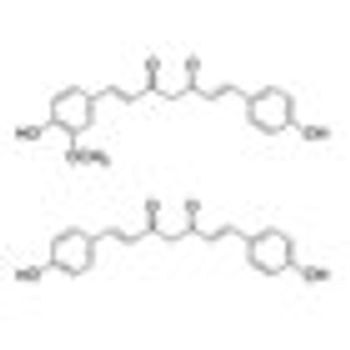
Addition of curcuminoids to treatment with docetaxel was well tolerated and showed promise in improving the response rate to docetaxel “in terms of both PSA decrease and objective response” in a phase II trial in patients with castration-resistant prostate cancer.
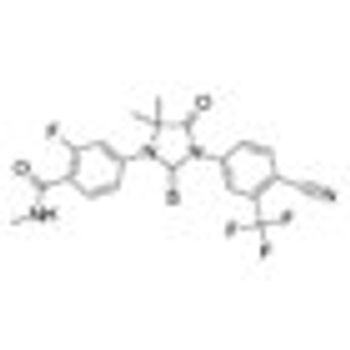
Monotherapy with enzalutamide (Xtandi) achieved a “high PSA response rate and marked PSA decline” in patients with hormone-naïve prostate cancer after 6 months in a single-arm, multicenter phase II study.
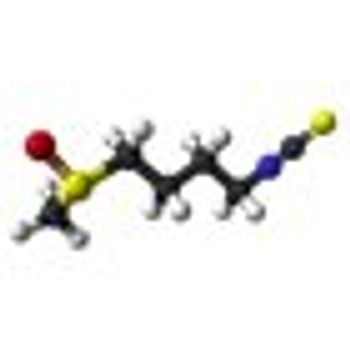
Treatment with 200 µmol per day of sulforaphane for 20 weeks was “feasible, safe,” and inhibited histone deacetylase (HDAC) function in a single-arm study of 20 patients who had non-castrate biochemical (PSA)-recurrence of prostate cancer despite surgery or radiation.

Ahead of the 2013 ASCO meeting we highlight some of this year's prostate cancer sessions, many of which focus on how best to use the new agents that have been approved recently, as well as looking into new drugs and combinations presented from early trials.

The AUA recently released its first set of treatment guidelines addressing the treatment of men with metastatic castration-resistant prostate cancer (mCRPC) at its 2013 Annual Meeting. The guidelines were released to address the increasingly complex treatment landscape available for patients with mCRPC.

Survival data of prostate cancer patients 70 and older show that those with three or more comorbidities and low- or intermediate-risk prostate cancer are less likely to die from prostate cancer than another health issue. But those with aggressive, high-risk disease are more likely to die from their prostate cancer.
Palliative radiotherapy is an effective means of alleviating pain and improving overall quality of life in elderly patients with bone metastases, according to a new study.
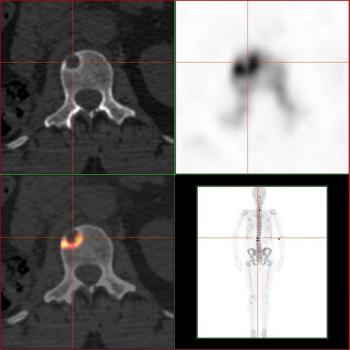
This slide show features images of diffuse osteoblastic bone metastases in a 70-year-old advanced prostate cancer patient, bone metastases in the vertebral column of a kidney cancer patient, and more.

The American Urological Association (AUA) released a new clinical guideline detailing recommendations for the use of prostate cancer screening in average-risk men based upon evidence from a systematic literature review. The guideline, which was announced during the 2013 AUA Annual Meeting, recommends that men aged 55 to 69 years who are considering undergoing prostate cancer screening should talk with their physicians about the benefits and risks of screening.
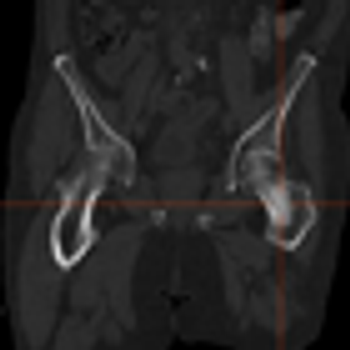
Radium-223 dichloride (Xofigo) received FDA approval for the treatment of symptomatic metastatic castration-resistant prostate cancer that has metastasized to the bone but no other organs.

The majority of patients with systemic prostate cancer treated with androgen deprivation therapy (ADT) will develop castration-resistant prostate cancer (CRPC).
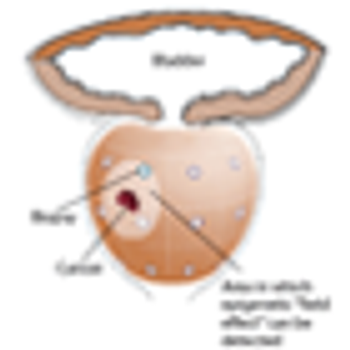
A 46-year-old man sought consultation for an abnormal prostate-specific antigen (PSA) level of 9 ng/mL and one prior negative biopsy. Five months ago, while traveling, he had presented to an urgent care facility with a 24-hour history of fever, chills, nausea, and vomiting.
A cohort-based study found that men with prostate cancer who took cholesterol-lowering statins had a lower risk of dying from their prostate cancer.