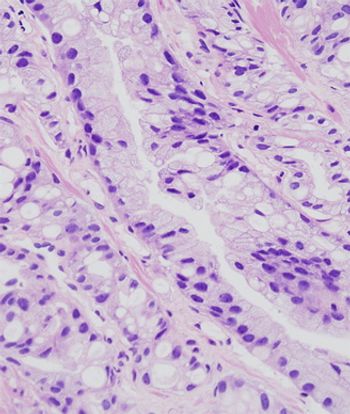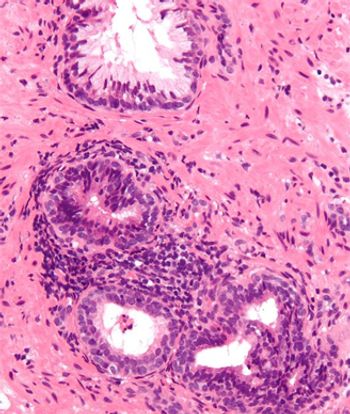
Markers of inflammation should be analyzed and reported in prostate biopsies, according to the results of a new study. Researchers found that negative prostate biopsies that had markers of inflammation were less likely to be diagnosed with prostate cancer in a subsequent prostate biopsy.