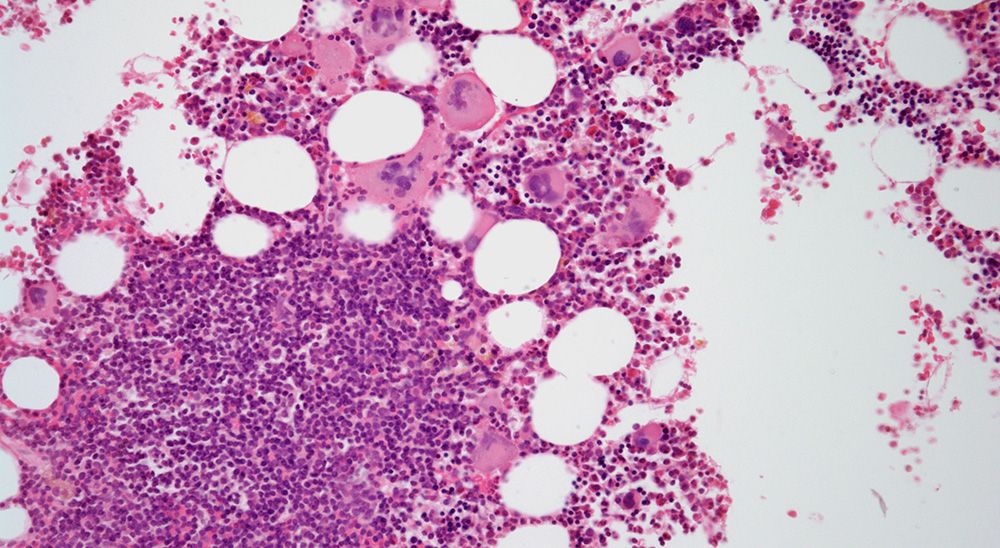
Myeloproliferative Neoplasms
Latest News
Video Series
Latest Videos
Podcasts
CME Content
More News

John O. Mascarenhas, MD, led a panel discussion on myelofibrosis management including the use of JAK inhibitors and transplantation.

Patients with myelofibrosis and anemia can now receive treatment with momelotinib following the agent’s approval by the FDA based on results from the phase 3 MOMENTUM trial.

Mohammad Bakri Hammami, MD, highlights a need to address socio-racial disparities among Black and non-Black patients with primary myelofibrosis to ensure that everyone receives high-quality treatment.

Selinexor plus ruxolitinib is under investigation as a treatment for JAK inhibitor-naïve patients with myelofibrosis as part of the phase 3 XPORT-MF-034 trial.

Data from the phase 2 MANIFEST study suggest that pelabresib plus ruxolitinib may improve the standard of care for patients with myelofibrosis who are naïve to treatment with JAK inhibitors.
Most treatment-related adverse effects were low-grade following treatment with BMS-986158 plus ruxolitinib or fedratinib in a population of patients with intermediate- or high-risk myelofibrosis.

The phase 3 LIMBER-304 trial examining parsaclisib with ruxolitinib vs matched placebo will be discontinued after an interim analysis concluded it would likely miss the primary end point.

An expert from the University of Texas MD Anderson Cancer Center says momelotinib may lead to decreased transfusion dependence vs danazol in myelofibrosis and intermediate- or high-risk anemia.
Tamibarotene is a first-in-class oral first-in-class selective retinoic acid receptor α agonist that is being evaluated in combination with azacitidine for newly diagnosed higher-risk myelodysplastic syndrome.

According to the phase 3 PERSIST-1 trial and PAC203 trial, thrombocytopenia was associated with myelofibrosis treatment.

Patients with myelofibrosis and anemia who were previously treated with a JAK inhibitor experienced durable responses up to 48 weeks with momelotinib.
The marketing authorization application for oral momelotinib in myelofibrosis has been approved by the European Medicines Agency.

Investigators believe that disease modifying agents may demonstrate benefit in patients with myeloproliferative neoplasms prior to allogeneic hematopoietic cell transplant.

Based on results from the phase 3 MOMENTUM trial, the FDA has accepted a new drug application of momelotinib for patients with myelofibrosis.

Navitoclax Plus Ruxolitinib Combo Elicits Responses Indicative of Myelofibrosis Disease Modification
Bone marrow fibrosis improvement and variant allele frequency reduction observed with navitoclax plus ruxolitinib for patients with previously treated myelofibrosis may suggest disease modification.

John Mascarenhas, MD, hosts a panel of experts who discusses current strategies used to stratify risk patients with myelofibrosis and their preferences for sequencing therapy.

Patients with intermediate- or high-risk primary or secondary myelofibrosis with a low platelet count may derive benefit from treatment with pacritinib following its accelerated approval by the FDA.

Topline findings from the phase 3 MOMENTUM study indicated that patients with myelofibrosis experienced a statistically significant reduction in symptoms following treatment with momelotinib.

At 24 weeks, patients with myelofibrosis transfused with serum ferritin and receiving momelotinib experienced a treatment response.

At ASH 2021, CancerNetwork® spoke with Ruben Mesa, MD, of UT Health San Antonio MD Anderson Cancer Center, about which presentation from the conference could be practice changing for patients with myelofibrosis.

At ASH 2021, CancerNetwork® spoke with Ruben Mesa, MD, of UT Health San Antonio MD Anderson Cancer Center, about FDA approvals for myelofibrosis that may impact the standard of care.

Using the International Prognostic Scoring System (IPSS) score and JAK2 mutation status, thrombosis risk could potentially be identified for patients with primary myelofibrosis.

At ASH 2021, CancerNetwork® spoke with Ruben Mesa, MD, of UT Health San Antonio MD Anderson Cancer Center, about what changes in the treatment of myelofibrosis stood out to him most.

At ASH 2021, CancerNetwork® spoke with Ruben Mesa, MD, of UT Health San Antonio MD Anderson Cancer Center, about big breakthroughs in the treatment of myelofibrosis over the preceding year.
Patients with cytopenic myelofibrosis produced a tolerable safety profile when receiving treatment with pacritinib.