
This phase III trial tested T-DM1 plus pertuzumab vs chemotherapy and dual HER2 blockade in patients with HER2+ breast cancer.

Your AI-Trained Oncology Knowledge Connection!


This phase III trial tested T-DM1 plus pertuzumab vs chemotherapy and dual HER2 blockade in patients with HER2+ breast cancer.
Researchers looked at patient-reported outcomes with partial breast vs whole breast irradiation in patients with breast cancer who were not receiving chemotherapy.
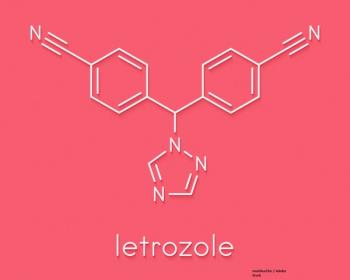
The GIM4 study looked at the effect of 2 to 3 years vs 5 years of letrozole on risk of recurrence in HR-positive early breast cancer.

Cancer Network spoke with Debu Tripathy, MD, Chair of Breast Medical Oncology at MD Anderson Cancer Center, on how ribociclib plus hormone therapy extends survival for patients with premenopausal advanced HR-positive breast cancer.

A large international study looked at whether pregnancy is safe after breast cancer for women who have a BRCA mutation.
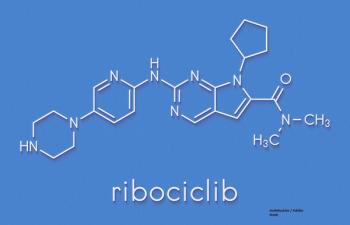
The MONALEESA-7 trial tested the combination of ribociclib with endocrine therapy in premenopausal women with advanced HR-positive/HER2-negative breast cancer.

Ahead of the ASCO Annual Meeting, we discuss the use of germline testing and PARP inhibitors in patients with breast cancer with Jennifer K. Litton, MD.

Ahead of the ASCO Annual Meeting, we discuss the use of genomic profiling to guide treatment decisions for ER+ breast cancer patients with Harold J. Burstein, MD, PhD.
Researchers tested a novel antibody-drug conjugate known as trastuzumab deruxtecan in an expansion cohort of a phase I study of patients with advanced HER2+ breast cancer previously treated with trastuzumab emtansine.

Researchers looked at whether eating a low-fat diet helped improve survival in breast cancer, in a study presented ahead of the ASCO Annual Meeting.

Cancer Network spoke with Ruth Oratz, MD, a medical oncologist at NYU Langone Perlmutter Cancer Center in New York City, about the use of PARP inhibitors for breast cancer.
Researchers tested whether certain patients with HER2-positive breast cancer may eventually be eligible for non-surgical management.
The results of a new study add to the list of benefits seen with exercise among breast cancer survivors: reducing the risk of cardiovascular disease.
Researchers evaluated whether breast cancer patients with hypothyroidism at diagnosis or who develop it later have an increased risk of recurrence or death.

The long-term results of the phase III HannaH trial confirmed the similarity between the subcutaneous and intravenous formulation of trastuzumab in patients with HER2+ breast cancer.
Researchers tested omitting chemotherapy from a treatment regimen involving dual blockade with pertuzumab and trastuzumab in patients with metastatic HER2+ breast cancer.
Researchers will discuss new findings on continuous vs intermittent chemotherapy schedules in advanced breast cancer at the ESMO Breast Cancer Congress.
Researchers tested whether TP53 mutation status can determine the function of estrogen receptor-beta (ESR2) in patients with triple-negative breast cancer.
A study of genetic testing in ovarian and breast cancer patients found substantial disparities in testing rates for ovarian cancer patients.
In this slideshow, various clinical guidelines on breast cancer screening are summarized, including the ACP's recent recommendations that have come under some scrutiny.

Dr. McArthur discusses the latest results of immunotherapy trials in the treatment of triple-negative breast cancer.

Cancer Network spoke with Hank Schmidt, MD, about the 10-year follow-up results of the EORTC AMAROS trial of radiotherapy vs surgery of the axilla in breast cancer patients with a positive sentinel node.
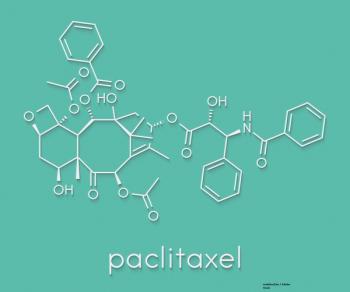
The combination of trastuzumab and paclitaxel 'represents an important step forward in de-escalating therapy' for HER2+ breast cancer.
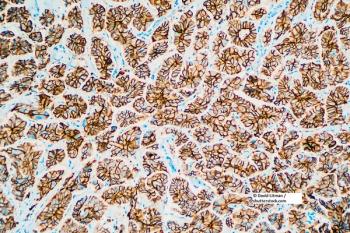
A genomic and proteomic analysis of HER2+ breast cancer cell lines that are resistant to trastuzumab found a deregulation of the cell death pathway known as TRAIL.
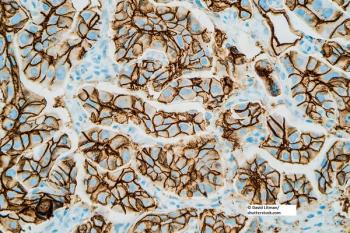
Researchers tested whether the use of alternative control probes to classify HER2 status in breast cancer could lead to substantial false positives.