Kidney Cancer
Latest News
Latest Videos

CME Content
More News
In patients with metastatic renal cell carcinoma, neoadjuvant immunotherapy–based combinations yielded reductions in tumor size and pathologic necrosis at the time of cytoreductive nephrectomy.
Clear cell renal cell carcinoma is driven by a metabolic switch that decreases VHL and increases ATP, according to an expert from Vanderbilt University Medical Center.
Findings from the phase 3 KEYNOTE-564 trial support adjuvant pembrolizumab following nephrectomy as a standard-of-care treatment for patients with clear cell renal cell carcinoma.
The improvements occur regardless of PD-L1 status among patients receiving lenvatinib plus pembrolizumab for advanced renal cell carcinoma in the phase 3 CLEAR study.
Using a laser ablation system, imaging mass cytometry is designed to measure over 40 metal isotopes in a tumor sample to identify how immune cells in clear cell renal cell carcinoma are organized.
Brian Rini, MD, reviews data from the 5-year analysis of the KEYNOTE-426 trial in advanced clear cell renal cell carcinoma.

Lenvatinib Plus Pembrolizumab Versus Sunitinib in Patients With Advanced Renal Cell Carcinoma (aRCC)
Robert Motzer, MD, presents the overall survival analysis data from the 4-year follow-up of the CLEAR study in patients with advanced renal cell carcinoma.

Experts present and discuss new findings from the CheckMate 9ER trial, evaluating nivolumab plus cabozantinib vs sunitinib in renal cell carcinoma.

A panel of experts, a patient, and her caregiver discuss her renal cell carcinoma diagnosis, the prevalent treatment options, and optimal strategies for patient education.
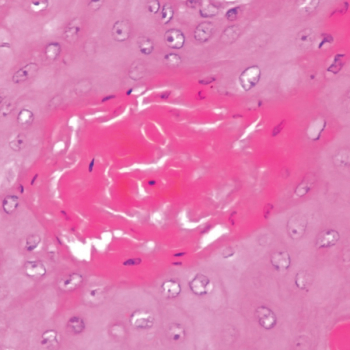
Syed Arslan Shehzad Shah, MD, and colleagues present findings from a broad investigation into this rare neoplasm of the kidney, including data on patient demographics and mean survival.
Kirollos S. Hanna, PharmD, BCPS, BCOP, FACCC, offers a perspective on a clinical quandary published recently in the journal ONCOLOGY.
Data from the phase 2 KEYNOTE-B61 trial indicate that pembrolizumab plus lenvatinib’s safety profile in the first-line treatment of patients with non-clear cell renal cell carcinoma is safe and manageable.

Pembrolizumab plus axitinib also improves the overall response rate vs sunitinib alone among patients with clear cell renal cell carcinoma in the phase 3 KEYNOTE-426 trial.

Data from the phase 3 CONTACT-03 trial highlight the necessity of randomized, prospective assessment of re-challenge with checkpoint inhibitors in renal cell carcinoma, according to an expert from Dana-Farber Cancer Institute.
Lenvatinib plus pembrolizumab generates no new safety signals among patients with advanced renal cell carcinoma in the phase 3 CLEAR study.
Patients with renal cell carcinoma with variant histologies appear to benefit from treatment with a triplet regimen of cabozantinib, nivolumab, and ipilimumab.
The combination is also associated with a manageable toxicity profile in the second and subsequent treatment lines for patients with advanced renal cell carcinoma.
Santosh Rao, MD, discusses ongoing and potential future research efforts aiming to maximize the benefits that patients with kidney cancer may derive from integrative oncology treatment techniques.

Santosh Rao, MD, discusses guideline recommendations and therapeutic techniques that underscore the current integrative oncology landscape for patients with kidney cancer.
The addition of cabozantinib to nivolumab and ipilimumab also appears to improve responses in patients with advanced renal cell carcinoma in the phase 3 COSMIC-313 trial.

Chung-Han Lee, MD, PhD, and colleagues discuss the case of a patient with renal cell carcinoma, touching on optimal treatments, toxicities, and other factors.

A panel of experts discuss the updated results of the phase 3 CLEAR trial presented at the 2022 International Kidney Cancer Symposium.

Data from the phase 3 ZIRCON trial evaluating 89Zr-DFO-girentuximab in patients with renal tumors may be practice changing, according to an expert from the University of California Los Angeles.

According to an expert from University Hospitals, integrative oncology has a place in the treatment of patients with kidney cancer alongside palliative care, psycho-oncology, and physical therapy.

Pharmacy director Kirollos Hanna, PharmD, BCPS, BCOP, FACC, discusses how to navigate shared toxicities between combination immunotherapy and VEGF inhibitors for patients with renal cell carcinoma.