Patients with low-risk myelodysplastic syndrome saw more durable responses and higher tolerability when taking luspatercept-aamt compared with the placebo.

Your AI-Trained Oncology Knowledge Connection!


Patients with low-risk myelodysplastic syndrome saw more durable responses and higher tolerability when taking luspatercept-aamt compared with the placebo.

Patients with myelodysplastic syndromes showed favorable event-free survival with allogeneic stem cell transplant compared with those being treated with continuous 5-azacytidine.
The combination of venetoclax and azacitidine will be considered as a treatment for patients with newly diagnosed, higher-risk myelodysplastic syndrome.
Data presented from the phase 3 SIMPLIFY 1 and SIMPLIFY 2 trials at 2021 EHA indicate success of momelotinib in extending overall survival in patients with myelofibrosis who achieve transfusion independence.
A study published in The Lancet Hematology found an increased risk of developing myelodysplastic syndrome and acute myeloid leukemia when patients with cancer were treated with PARP inhibitors compared with placebo.
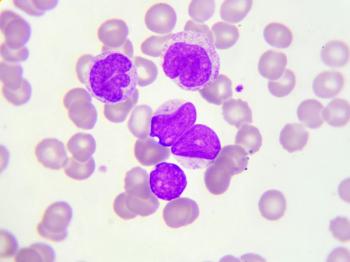
Data from initial dose cohorts of a phase 1/2 trial indicated the agent was found to safely drive natural killer cell proliferation in patients with high-risk myelodysplastic syndromes and acute myeloid leukemia.

The FDA granted breakthrough therapy designation to magrolimab for the treatment of newly diagnosed myelodysplastic syndrome.
The study evaluated the efficacy and safety of IV rigosertib in patients with high-risk MDS who had progressed on, failed to respond to, or relapsed after previous treatment with an HMA within 9 cycles over the course of 1 year after initiation of HMA treatment.

Takeda Pharmaceutical Company Limited announced the FDA granted pevonedistat, its investigation NEDD8-activating enzyme inhibitor, breakthrough therapy designation to treat patients with higher-risk myelodysplastic syndrome.

The FDA has approved an oral combination of decitabine and cedazuridine for adult patients with myelodysplastic syndromes.

The FDA approved the first and only erythroid maturation agent for the treatment of anemia in patients with lower-risk myelodysplastic syndromes.
The study, published in JAMA Oncology, examined whether treatment of 22 solid tumor types was associated with two therapy-related conditions.
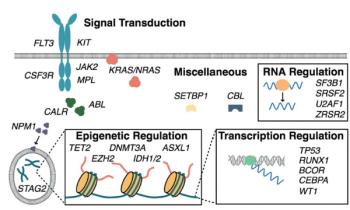
In this first part of our two-part review, we introduce mutation profiling as a relevant clinical tool for hematologists treating patients with myeloid malignancies.

Although there are currently no approved second-line therapies for high-risk MDS, researchers have discovered that rigosertib has the potential to benefit this population of patients.

A new study demonstrates that it is possible to vaccinate patients with MDS against a decitabine-induced antigen and that the level of induced expression is sufficient to trigger cytotoxicity in patient-derived vaccine-induced T cells.

A combination of the FLT3 kinase inhibitor quizartinib with 5-azacitidine or low-dose cytarabine is active in patients with FLT3-ITD mutated myeloid leukemias, according to a new study.

This video reviews novel treatment approaches in the management of higher-risk myelodysplastic syndromes.

Researchers presented data at the 2017 ASH annual meeting about their new personalized MDS prediction model that uses clinical and genomic data to help better guide therapy and improve outcomes compared with other models.

Patients with myelodysplastic syndrome undergoing a reduced–intensity conditioning regimen prior to allogeneic stem-cell transplantation had similar 2-year survival outcomes as patients who underwent myeloablative conditioning.

This video explores the current treatment landscape for lower-risk MDS patients, including the various agents and sequencing strategies employed to best optimize outcomes.

The use of the thrombopoietin receptor agonist eltrombopag was clinically effective at raising platelet counts in patients with lower-risk myelodysplastic syndromes with severe thrombocytopenia.

This look ahead at hematologic malignancies in 2017 focuses on new agents being studied for the treatment of acute leukemias, myelodysplastic syndromes, and chronic lymphocytic leukemia.

This video examines different mutational profiles of therapy-related myeloid neoplasms and how they can affect approaches to treatment.

Patients with AML or MDS with unfavorable risk cytogenetic profiles, TP53 mutations, or both had good clinical response and robust mutation clearance with decitabine.

A scoring system including several patient characteristics was found to have significant prognostic value for patients with myelodysplastic syndromes undergoing allogeneic hematopoietic cell transplantation.