
John Kirkwood, MD, PhD, provides an overview of the treatment options for patients with melanoma.

Your AI-Trained Oncology Knowledge Connection!


John Kirkwood, MD, PhD, provides an overview of the treatment options for patients with melanoma.
Patients with previously untreated metastatic uveal melanoma who also harbored HLA-A*02:01 and were treated with tebentafusp experienced a longer overall survival compared with the control group of a phase 3 clinical trial.

A subset of patients with metastatic uveal melanoma achieved promising responses after treatment with entinostat and pembrolizumab.
Patients with relapsed/refractory unresectable or metastatic melanoma may derive benefit from alrizomadlin, which has received a fast track designation from the FDA.
Previously treated patients with HLA-A*02:01-positive metastatic uveal melanoma saw survival prediction improvements with ctDNA when compared to RECIST 1.1.

The use of adjuvant pembrolizumab resulted in a recurrence-free survival benefit for patients with resected high-risk stage II melanoma.
Patients with previously untreated metastatic or unresectable melanoma achieved promising benefit following treatment with relatlimab/nivolumab combination therapy.
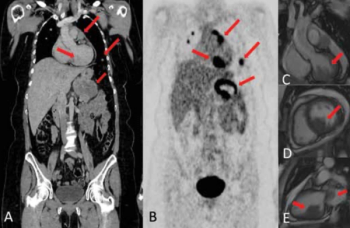
This clinical quandary discusses oligoprogressive disease in metastatic melanoma and how treatment with immunotherapy and targeted therapy affect the disease.

Tebentafusp has demonstrated promising responses in metastatic uveal melanoma, leading to FDA and European Medicines Agency approval of a biologics license application and marketing authorization application for the agent.

Gut microbes may help to predict adverse effects and outcomes in patients with advanced melanoma who are being treated with dual immune checkpoint inhibitors.

The results from a phase 1 trial that focused on a second cohort of patients with unresectable or metastatic melanoma identified positive safety and promising topline survival outcomes when treated with the combination of UV1 and pembrolizumab.

According to the results of the phase 2 KEYNOTE-629 trial, patients with locally advanced or recurrent/metastatic cutaneous squamous cell carcinoma appear to receive promising anti-tumor benefit from pembrolizumab.

Pembrolizumab met its primary end point of prolonged recurrence-free survival in the phase 3 KEYNOTE-716 trial for patients with stage II resected high-risk melanoma.

Immunotherapy agent nemvaleukin alfa was granted an FDA fast track designation for the treatment of patient’s mucosal melanoma for previously undergone treatment with an anti-PD-L1 therapy.

Cemiplimab for patients with either metastatic or locally advanced basal cell carcinoma showed antitumor activity in a phase 2 trial.

Patients with PD-1 inhibitor-resistant melanoma who were treated with ipilimumab plus anti–PD-1 therapy saw significantly better long-term responses than those on ipilimumab alone.

Bempegaldesleukin plus nivolumab demonstrated positive antitumor activity while maintaining a tolerable safety profile in the PIVOT-02 trial for patients with previously untreated metastatic melanoma in the first-line setting.

Pembrolizumab has been granted an expanded indication by the FDA for locally advanced cutaneous squamous cell carcinoma.

Targovax announced the FDA granted fast track designation to its investigational agent ONCOS-102 for PD-1–refractory advanced melanoma.
With further follow-up, lifileucel (LN-144) continued to show durable responses in patients with heavily pretreated advanced or metastatic melanoma who have progressed on multiple therapies, including prior anti–PD-1/PD-L1 therapy
In an updated analysis of the combination of lenvatinib and pembrolizumab for patients with advanced melanoma efficacy results continue to show a durable response.

Nivolumab monotherapy, or in combination with ipilimumab, continued to demonstrate durable improvements in overall survival compared with ipilimumab alone in patients with previously untreated advanced melanoma.
About 40% of patients who progressed on placebo following resection of melanoma experienced an objective response with crossover to pembrolizumab in the phase 3 EORTC 1325/KEYNOTE-054 trial.
A higher overall response rate was noted with lifileucel and pembrolizumab versus pembrolizumab alone in patients with checkpoint inhibitor–naïve advanced melanoma, according to phase 2 trial results that were reported at the 2021 ASCO Annual Meeting.

Progression-free survival more than doubled when relatlimab was added to nivolumab to treated patients with previously untreated, unresectable or metastatic melanoma.